A Pathogen's Guide to the Nose (Review)
Part III: At Home Nasal Remedies & The Coming Winter
At Home Nasal Interventions
In my paid only Substack post I posted about therapeutics and prophylactics that could be administered intranasally. However, I ran out of space in that post (apparently 10 pages worth is the limit!) and so I have taken portions of that post and am posting it here, which works out since many of these may be more applicable to many of you.
Some of these are easy to obtain in any drug store as well as widely available online and could thus be adopted by millions of people very easily. Nonetheless, It is important to consult a medical expert before trying any of these interventions.
Here I will outline 3 common therapeutics and the evidence for some of these compounds.
Iodine
Iodine has been known to have broad spectrum antimicrobial properties and is well known for its topical usage for IV needles and cuts and wounds. It’s antimicrobial mechanism of action occurs through iodination of lipids and oxidation of cytoplasmic and membrane compounds that leads to cell death. If Iodine can be administered via the nasal or oral route then it could provide a cheap, noninvasive approach for treatment of COVID.
Taken from Kontos, Z. 2021 (emphasis mine):
Intranasal saline sprays containing elemental iodine as an active ingredient has been used for nasal moisturizing and prevention and/or treatment of sinusitis or rhinitis [10]. Iodine-containing intranasal/oral moisturizing saline sprays are being explored as drug agents against coronaviruses. Their potential use has been supported by a recent clinical safety trial that demonstrated that intranasal Povidone-Iodine (PVP-I; 1.25%) spray had no adverse effects for up to five months and therefore, could be used against coronaviruses (SARS-CoV-1/2 and Middle East Respiratory Syndrome—MERS) [11]. Regarding the antiviral efficacy of iodine-containing nasal/oral sprays, nasal and oral formulations containing PVP-I have been reported to inactive SARS-Cov-2 in vitro [12, 13]. Thus, PVP-I oral antiseptic is potentially efficacious in reducing the transmission risk of coronaviruses in dental practice [13]. SARS-CoV-2 is internalized into the host cells via two viral receptors of host cell infection: Angiotensin-Converting Enzyme 2 (ACE2) and CD147 (a highly-glycosylated transmembrane protein) by binding to them using virus spike proteins (SP) [14]. The mechanism of action of PVP-I mouth rinse/nasal spray involves targeting of the host’s ACE2 for inhibition. It diminishes the ACE2 receptors in lymphocytes by promoting their absorption from host epithelial tissue [9]. This reduces the concentration of SARS-CoV-1/2 shed in saliva and nasal fluid.
Unfortunately, the data in this area is sparse, and there is some indications that intranasal administration of Iodine may not be effective.
Iodine-V
Iodine-V is a fulvic acid/iodine complex, and is usually labelled as fulvic iodine. In an in vitro study by Kontos, Z. 2021 cells infected with SARS-COV2 were treated with Iodine-V and showed a significant reduction in viral load. However, because this study was not done in a clinical setting or in vivo the results should be taken with heavy skepticism until more studies are conducted. As of now there is no strong evidence in support of nasal administration of Iodine-V being beneficial.
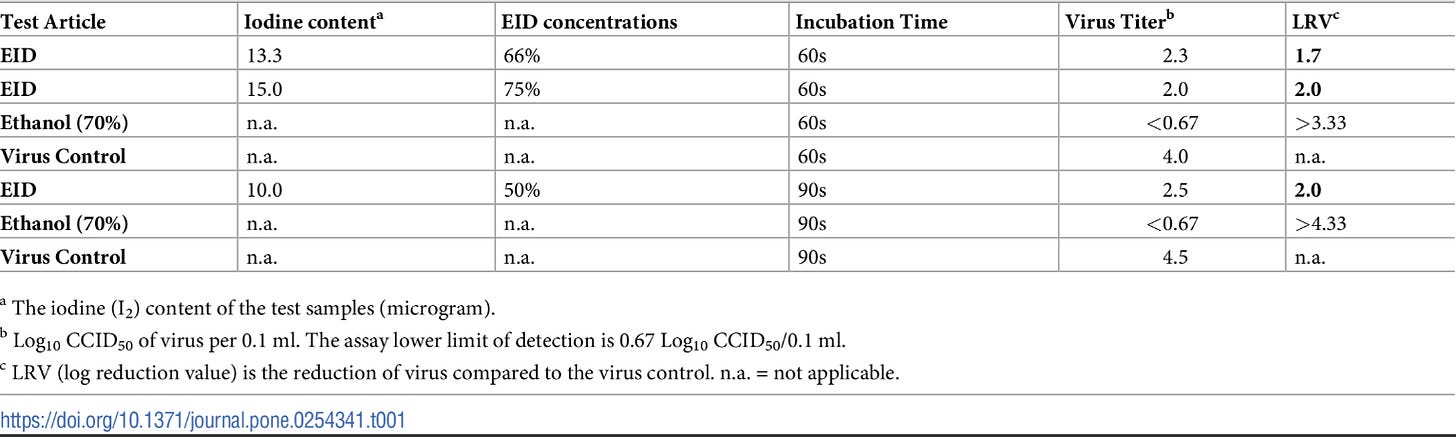
Providone-Iodine
Providone-Iodine is an iodophore that contains water soluble iodine as well as polyvinylpyrrolidone (PVP) as a carrier agent, and is one of the most widely used antiseptics. Its use as a therapeutic against COVID would therefore provide an easy, cheap, therapeutic/prophylaxis. Similar to the Kontos, Z. 2021 study Providone-Iodine has shown to be strongly efficacious against SARS-COV2 in vitro. However, clinical studies have indicated mixed results.
A study by Guenezan et. al. 2021 in which researchers used Providone-Iodine mouthwash, gargle, and nasal spray found no significant reduction in viral load compared to a control group over the course of the study, and there was a large number (42%) of people within the experimental group that noted thyroid dysfunction.
However, a study by Seet et. al. 2021 indicated that oral Providone-Iodine reduced COVID infection significantly within a prophylactic setting.
Iodine administered in the form of either Iodine-V or Providone-Iodine in an in vitro setting has shown high effectiveness, supported by their common usage as a topical antiseptic, however this does not seem to translate over to a clinical, intranasal setting well based on some the studies out so far. Comparing these 2 studies Iodine administration may be most beneficial when administered as a prophylactic as the lower viral load may be easier to treat. More evidence about Iodine’s effective would greatly support at-home treatment options, although the concern about excessive Iodine should be looked into as well.
Carrageenan
Carrageenans have found greater broad scale usage in recent years, and its nasal spray form is best known as betadine (Correction- Betadine is sold as both a providone-iodine spray as well as a carrageenan spray). As indicated by Koenighofer et. al. 2014:
Carrageenans belong to a family of linear, sulfated polysaccharides which are found in some species of red seaweed. Food, cosmetic, and pharmaceutical industry use carrageenans extensively as emulsifying and binding agent for products like ice cream, various gels, toothpaste and others [6]. But carrageenans also revealed antiviral activity against a range of animal viruses [7] and are even used to prevent sexual transmitted viral infections as a component of spermicides [8]. Furthermore, in vitro and in vivo studies have recently shown that carrageenans are potent inhibitors of papilloma virus [9], human rhinovirus [10], influenza A virus [11], respiratory syncytial virus and also of human enterovirus 71 [12].
Carrageenan has a unique mechanism of action. Its utility as a thickener and emulsifier allows it to form a coating when sprayed in the nose and throat. This coating essentially creates a shield that prevents adherence of pathogens to the mucus and thus does not allow entryway into host cells.
As noted in a review by Stathis et. al. 2021 (emphasis mine):
Iota-carrageenan is nontoxic and works by creating a physical barrier against viruses in a nonclinical study of the compound [75]. The compound has also been reported to be effective against the common cold compared with placebo in clinical trials, making it a good potential candidate for reducing transmission of SARS-CoV-2 [76]. In an analysis of two randomized controlled studies of iota-carrageenan against the common cold, iota-carrageenan was shown to be more effective than placebo at reducing the duration of symptoms in patients infected with human coronavirus by 3.9 days on average in one trial (p < 0.01) and 3.1 days in the other trial (p < 0.01) [77]. Iota-carrageenan has been reported to be effective against SARS-CoV-2 in vitro alone and in combination with xylitol [19]. There currently is no published in vivo data, but iota-carrageenan is being investigated in a clinical trial as a preventative measure against COVID-19 [52].
Unfortunately, there is no direct clinical evidence of carrageenan against SARS-COV2, but there has been evidence of carrageenan being effective against the common cold, and if the mechanism of action depends upon its ability to create a barrier against viral entry, it would make sense to argue this should have an affect against SARS-COV2.
As summarized in Moakes et. al. 2021, in which researchers formulated a carrageenan-based nasal spray for a possible prophylactic usage and found some in vitro efficacy (emphasis mine):
This study reports on a nasal formulation with the capacity to combat such challenges, focusing on severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Formulation of a polysaccharide-based spray, known for its mucoadhesive properties, is undertaken and it is characterized for its mechanical, spray distribution, and antiviral properties. The ability to engineer key mechanical characteristics such as dynamic yield stresses and high coverage is shown, through systematic understanding of the composite mixture containing both gellan and λ-carrageenan. Furthermore, the spray systems demonstrate highly potent capacities to prevent SARS-CoV-2 infection in Vero cells, resulting in complete inhibition when either treating, the cells, or the virus, prior to challenging for infection. From this data, a mechanism for both prophylaxis and prevention is proposed; where entrapment within a polymeric coating sterically blocks virus uptake into the cells, inactivating the virus, and allowing clearance within the viscous medium.

Carrageenans may provide for a cheap, effective method of prophylactic protection against SARS-COV2, although their over the counter availability may be difficult to obtain. There also may be some concern about the accumulation of carrageenan causing nasal obstruction through the thickening mucosal layers in the nose. However, the ability to have an easily accessible, noninvasive prophylactic with very low side effects would be greatly beneficial for large scale prevention of SARS-COV2 infection. Although there are no published clinical trials against SARS-COV2, there is good enough evidence that may warrant adopting its usage.
Hypertonic Saline Sprays
Hypertonic saline (high salt) sprays may be one of the most ubiquitous household items out there. Many people have most likely used it to irrigate their noses from allergens, or to help moisten dry noses during the colder months. However, there is evidence that many of these nasal sprays may contain antiviral properties, and may prove very useful as a prophylaxis against viral infections.
Saline sprays are usually composed of salt water, although some of them may contain other ingredients such as baking soda (sodium bicarbonate). There are a few possible mechanisms of actions with salt solutions. The high salt levels can alter ion gate functions in specific cells, or may contribute to the osmotic pressure of infected cells. The chloride ions themselves, when uptaken by certain cells, may also be used for formation of sodium hypochlorite (bleach) which may be the active antiviral agent.
And here is some more elaboration taken form Stathis et. al. 2021 (emphasis mine):
Several randomized controlled trials have shown the efficacy of salt water in acute upper respiratory tract infections [30]. A small randomized controlled trial in the UK reported that saline irrigation and gargling reduced the duration of viral upper respiratory tract infections by 1.9 days (p = 0.01), reduced the transmission to household contacts by 35% (p = 0.006), reduced the use of over the counter medications by 36% (p = 0.004). More individuals in the intervention arm reduced viral shedding by >0.5 log10/day compared with controls (p = 0.04) [31]. The mechanism for the efficacy of saline against various viruses in the upper respiratory tract is that increased availability of local chloride ions (from NaCl) supports the production of hypochlorous acid (the active ingredient in bleach), a potential innate immune mechanism in epithelial cells [30,32]. The addition of NaCl to the cell culture medium inhibited various human viruses in a dose-dependent manner. Human coronavirus 229E replication was inhibited by saline concentrations between 30 and 50 mM [32]. Importantly, the entry of Cl- ion into the cells and its conversion by a peroxidase to hypochlorous acid was found to be necessary for the observed antiviral activity [32]. The data did not support any other mechanism (i.e., virucidal, adsorption inhibition). A study investigating the effect of hypertonic saline on SARS-CoV-2 in vitro, available on the preprint server, showed that 210 mM NaCl (1.2%) inhibited virus replication by 90% (p < 0.0005) and 260 mM NaCl (1.5%) inhibited viral replication by 100% (p < 0.0005) in Vero CCL-81 cells when compared with 110 mM NaCl (0.6%) [33]. The concentration of NaCl in the mucus layer found in the respiratory tract measured by in vivo tests has been reported to be in a range of 110 mM (0.6%)–130 mM (0.76%) [34,35]. Any NaCl solution with a concentration >130 mM that is applied to the nasopharynx would result in a net increase in chloride ions and sodium ions in the mucus layer.
Most studies with saline sprays have been conducted in conjunction with the common cold, and there is good evidence of saline sprays reducing the duration of symptoms in those infected with the common cold.
In an open labelled, pilot study conducted by Ramalingam et. al. 2019, participants within the intervention group were administered hypertonic saline solution for nasal irrigation and gargling (HSNIG), with the researchers noting shorter recovery and lower viral count and reduced need for over the counter medications (OTCM):
In this pilot, HSNIG significantly reduced the duration of URTI, OTCM use and illness within the household. A greater fall in viral shedding possibly explains the reduction in duration of symptoms and in symptomatic household contacts. This is in keeping with the lab evidence that cells utilise NaCl to mount an antiviral effect. A larger study powered for clinical and virological end-points is urgently needed to confirm these findings.
Fortunately, there have been some in vitro studies conducted on hypertonic saline solutions against SARS-COV2.
In a recent in vitro study conducted by Machado et. al. 2021, researchers indicated that hypertonic saline solutions inhibited SARS-COV2 infection in lung and kidney cells, and the authors proposed a different mechanism of action for the viral inhibition.

This in vitro study indicates an interesting mechanism of action, but because it was done in lung and kidney cells it would be difficult to create a high salt environment in the lungs through the use of nasal sprays, and large ingestion of salt would be detrimental to blood pressure. However, if this mechanism works with mucosal epithelial cells this treatment/prophylaxis should be effective.
Saline solutions are one of the most cheap and easily accessible over the counter remedies available. It is also very easy for people to create at home and administer themselves through the use of a neti pot.
Caution should be taken when done at home. Note that many of these studies use a hypertonic solution, meaning that the solutions contain a higher salt concentration relative to the typical ion levels in the nose. Therefore, saline sprays and irrigation should contain a higher salt concentration, although extremely high salt concentrations may end up becoming damaging to the nasal environment. Also, improperly cleaned items such as neti pots may become reservoirs for fungi and bacteria that may cause infections when used. Care should be taken to follow proper irrigation procedures.
Regardless, saline sprays and irrigation may be one of the cheapest, easily accessible ways of preventing and treating COVID infections. Not only can they be done quick and easily, there are hardly any notable downsides to chronic saline usage. More importantly, saline spray may help moisten dry noses, which could help to improve the nasal environment, and are especially important during the winter months where seasonal respiratory infections run rampant.
Winter is Coming

The examples listed above are some of the most common non-pharmaceutical interventions that many people may have access to. Although some of these, such as the iodine therapies, may not be beneficial in reducing SARS-COV2 infection, they may be some of the easiest items to obtain, especially the saline sprays. Also, many of these items do not require a prescription, so many people do not have to go through the bureaucratic procedures that have prevented people from being prescribed ivermectin or hydroxychloroquine as prophylaxis. More importantly, nasal interventions tend to be topical and are easier to administer without severe adverse events, and may provide direct antiviral action right at the site of infection, which can greatly reduce infection and viral load.
I’ll end this post with a warning that winter is coming (yes, very cliché) in the Northern hemisphere, and with winter comes very low humidity and low temperatures, all of which create a susceptible nasal environment; a dry environment removes the protective, moist environment in the nose while low temperatures reduce cilliary functions and inhibit the movement of debris and pathogens from the nose.

It also leads to social and behavioral changes; we are less likely to stay outside for long periods, and may spend even greater time indoors with family. One of the reasons I speculated that COVID may have taken hold so well at the beginning of the pandemic may have been that shutdowns caused many people to either shuffle from extremely crowded grocery stores to their homes where they stayed most of the day, and we know full well now that a majority of COVID infections occur inside the home between family members.
With the encroaching colder weather comes greater seasonal infections, and we should be well prepared to protect ourselves against infections, and non-pharmaceutical interventions may be one of the best, widely available ways of protecting against COVID and other diseases.
Additional Information
Olfactory Dysfunction Therapies
For those who are unaware, there are treatments for persistent anosmia and olfactory dysfunction. One easy, at home option is called olfactory training, where you smell strong odors such as citrus or other powerful odors a few times a day until the ability to smell comes back. This is probably a moot point though, since most people who lose their sense of smell may just challenge it by smelling strong odors anyways.
For those who have had persistent anosmia long after COVID recovery, there is some evidence that corticosteroids may help in reducing anosmia duration when paired with olfactory training.
In a study conducted by Kasiri et. al. 2021, researchers administered a corticosteroid called mometasone furoate along with olfactory training and found that the combination lead to greater improvements in severe, chronic anosmia than just olfactory training alone.
Although this study is promising, the administration of corticosteriods in nonsevere patients is heavily contentious, and there is not enough evidence at this time to fully recommend steroids to aid in anosmia.
However, this study does provide some evidence, and anyone who is experiencing chronic anosmia may want to discuss corticosteriod nasal sprays with their doctor.
Correction: Above I listed that Carrageenan goes under the name Betadine. However, that seems to be the company brand. Instead, the antiseptic/treatment option is the providone-iodine product while the cold protection item is comprised of Carrageenan (states Carragelose on the package).
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.


I would be very interested in your opinion on the nasal spray Xlear that is currently seeking an emergency use authorization with the FDA. Its ingredients are xylitol and grapefruit seed extract.