The nose is a magical structure. It provides us the ability to smell all manners of sweet goodness, whether it is the scent of desiccating leaves on an Autumn day, the smell of fresh baked goods pulled right from the oven, or even the smell of our favorite scented candles. But it also allows us to smell putrid things that may be harmful to us such as rotten milk, spoiled food, and backed up sewage.
Yes, the ability to smell has provided us so many benefits, and when we lose our sense of smell we lose all manners of external stimuli that help us navigate the world.
One of the most unique features of COVID-19 is anosmia, the loss of smell. It is one of the best ways to distinguish COVID from other respiratory illnesses. But what does the loss of smell mean, and could it indicate an important pathology for COVID infection?
This piece will provide a brief overview about our nasal passage, nasal infection by SARS-COV2, evidence of neuroinvasion, and why this may be responsible for the low infection & death rates seen in children.
The Nose: A Viral Entryway into the Body and Brain
I won’t go into too much detail here but I’ll outline the main takeaways.
The nose and the nasal cavity are part of the respiratory system, which helps with the exchange of gases such as CO2 and O2. When we inhale, we breathe in different gases as well as many irritants and pathogens. Because of this our nasal passage has been selected through evolution to respond to potentially dangerous exogenous compounds as well as aid in the inhalation process:
Nose hairs help to filter large bits of dust and pollen.
High levels of blood capillaries help to warm incoming air.
Mucus helps to moisten the air and trap dust and particulates.
The mucus membrane in general plays a large role in mediating immune responses to pathogens and allergens. Trapped pathogens can be disposed of via IgA antibodies that help to prevent pathogens from entering cells. There are also digestive enzymes called lysozymes that break down microbes.
Cilia, hairy organelles that comprise the epithelial cells of the nasal passageway, help to sweep mucus and trapped particles towards the pharynx where it will either be swallowed or spat out.
The nasal passageway is highly vascularized (high number of blood vessels) to help warm incoming air. It is also highly innervated (high number of nerves) which helps with sensing odors and other external stimuli. What’s unique about the nasal passage is that it contains a very thin, nearly nonexistent blood-brain barrier, meaning that passage into the central nervous system is much easier in the nasal passage than in other areas such as the spinal chord.
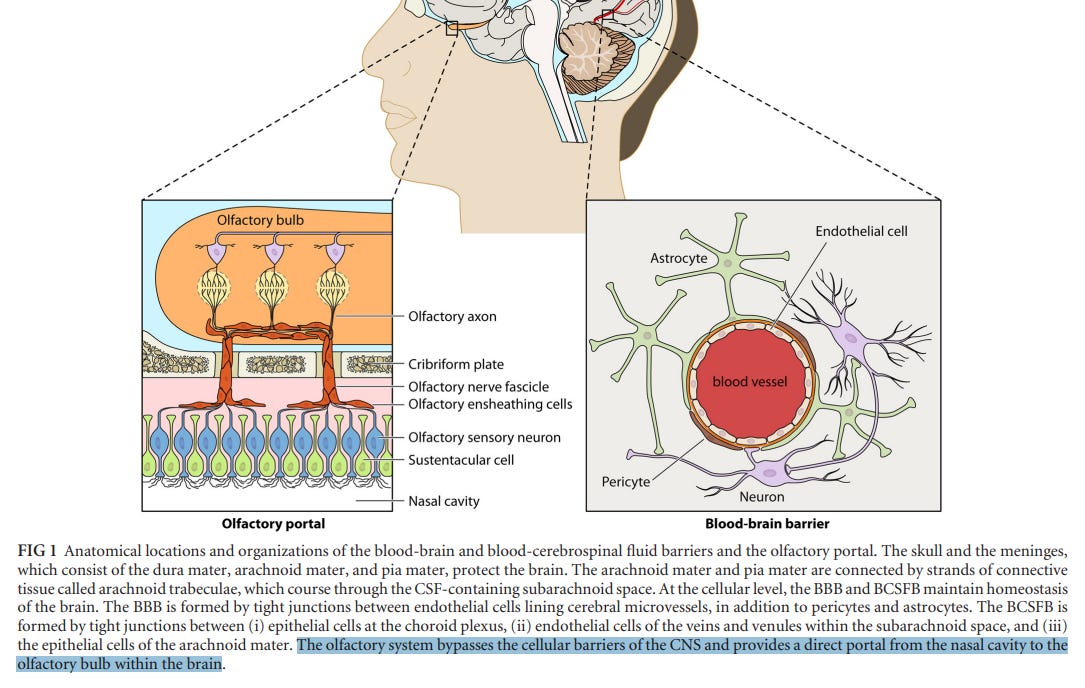
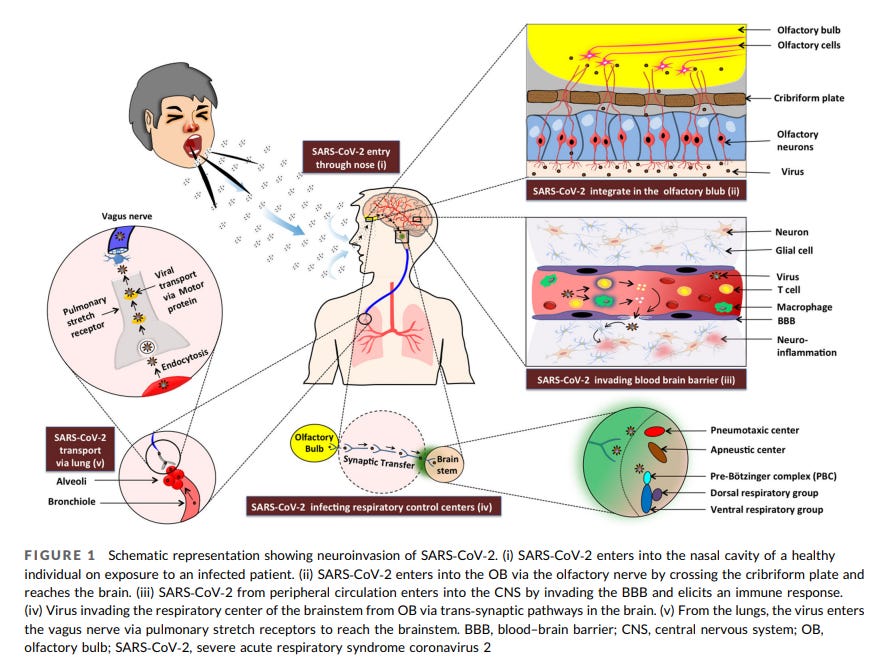
Unfortunately, the high vascularity, high innervation, and nearly absent blood-brain barrier makes the nasal cavity one of the best entryways for pathogens into the CNS and the body, making it a large area of concern when it comes to SARS-COV2 infection.
Neuroinvasion is actually not unique to SARS-COV2. There are many instances of bacteria, the flu virus, and even other corona viruses having the ability to invade the nervous system. However, the high level of anosmia seen in people infected with COVID indicates a need to examine the disproportionately high rate of occurrence with COVID and its relationship with the nervous system.
As noted in a review by Pérez et. al. 2020 (emphasis mine):
The CNS is vulnerable to viruses, and many of them end up reaching the brain such as the herpes virus, arboviruses, measles, influenza and HIV, among others. Coronaviruses can also act on the CNS [5-8] and this is why a pandemic with such a high number of affected could mean the appearance of neurological conditions. In fact, 36% of those affected by COVID-19 with neurological manifestations have been reported [9]. Coronaviruses can penetrate the CNS, affect both neurons and glial cells (a property known as neurotropism) and induce various neurological pathologies (neurovirulence) [6]. Regarding the similarity of SARS-CoV-2 with SARS-CoV-1 (causing the epidemic of 2006-2007), it is postulated that SARS-CoV-2 accumulates mainly in the nasal epithelium (neurotropism that would lead to anosmia) and the lower respiratory tract [6]. The appearance of early symptoms in the form of loss of smell (anosmia), imbalance and/or impaired gait (ataxia) and seizures should be considered as neurological manifestations of COVID-19 [7]. Specifically, the coronaviruses have been reported to cross the blood-brain barrier (BBB) [8], and three common virus routes to cross the BBB have been previously reported [9]: direct infection of endothelial cells that comprise the BBB; crossing permeable regions of the BBB and infection of cells that are licensed to cross that barrier, often referred as Trojan horse approach [10]. However, it is unlikely that SARS-CoV-2 can do so, due to the size of this virus, making it more likely to access via olfactory [11] or trigeminal nerves which would explain the anosmia prevalence in this pandemic.
The main hypothesis for SARS-COV2 neuroinvasion seems to be the direct infection of the CNS via the olfactory nerve.
As supported by Netland et. al. 2008 in which transgenic mice for the human ACE2 (hACE2) receptors were infected by SARS-COV and found to have neuronal damage. Although this study was conducted using SARS-COV both coronaviruses target the hACE2 receptor, and the implications can be contextualized to SARS-COV2 infections.
The authors noted:
Our results suggest that SARS-CoV primarily entered the brain via the olfactory nerve. However, the rate at which SARS-CoV spread within the brain was striking. Viral antigen was not detected until 60 to 66 h p.i. and, by this time, was already present in the olfactory bulb and several brain regions connected to this structure. Furthermore, 6 to 12 h later, viral antigen was detected throughout the brain and had spread to first- and second-order structures connected with the olfactory bulb as well as structures only remotely connected with the olfactory system. This is in contrast to other viral infections in the murine brain where spread from the olfactory bulb to directly connected sites takes approximately 1 day, with spread to more distal sites taking an additional several days (5, 6, 26, 37). One explanation for this rapid spread is that the replicative cycle of SARS-CoV is short…
Alternatively, virus load in initial sites of infection may be sufficiently high so that virus is able to spread transneuronally to distally connected neurons without a requirement for replication in all infected cells along a pathway.
So it seems there is strong evidence that not only is the nasal passage the main route of SARS-COV2 infection, it also indicates that CNS infection is highly likely and possibly plays a large role in the virus’ pathology.
Evidence of Neuronal Damage
Even people who have had mild COVID have downplayed the effects of anosmia. Although the symptoms of anosmia decline after a few weeks the loss of smell most likely indicates neuronal damage, and we have evidence of such damage.
A case presentation by Chiu et al. 2020 indicated a 19 year old female with persistent anosmia and a negative PCR test who showed a loss of olfactory bulb mass post COVID infection.
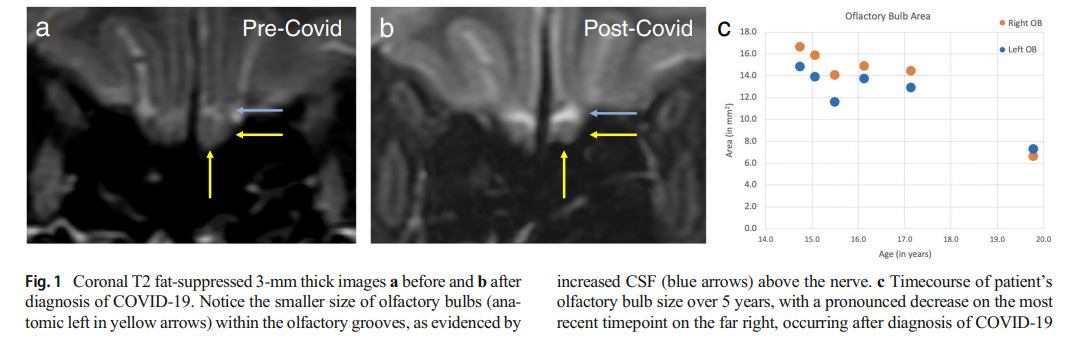
And this was further indicated in a study by Kandemirli et. al. 2020 in which 23 patients with persistent anosmia were imaged and many had olfactory bulb abnormalities.
The researchers concluded:
In this study, we evaluated 23 patients with persistent COVID-19 olfactory dysfunction. All patients were anosmic at the time of imaging based on TDI scores. We noted a high percentage of olfactory cleft opacification (73.9% of cases). There was reduction of olfactory bulb volumes, change in bulb shape and signal abnormalities. Some of the cases showed olfactory nerve filia clumping or scarcity. Additionally, 21.7% of cases had primary olfactory cortical signal abnormality.
Pathogenesis of olfactory dysfunction in COVID-19 disease is still incompletely understood, but there are some distinct features that separate this entity from other postviral olfactory dysfunction. Postviral anosmia in the setting of upper respiratory tract infection can account up to 40% of the cases, and is usually related to mucosal congestion and nasal obstruction (15). This can affect the airflow and impair the travel of odorants, despite an intact olfactory epithelium, and result in a conductive olfactory loss (4). On the other hand, there is no significant association with sinonasal symptoms in COVID-19 anosmia, as also shown in our cohort (16). This suggests that mechanisms other than sinonasal obstruction may play a role. Plausible mechanisms include direct damage to olfactory epithelium and olfactory bulb by the virus, and damage to olfactory epithelium secondary to inflammatory changes (4).
And lastly, a recent preprint from Douaud et. al. 2021 in which brain imaging before and after a COVID infection were compared for changes in grey matter thickness and intensity, with the study indicated a greater change in grey matter in post COVID patients. This indicates an important causal relationship between COVID infections and the presentation of neurocognitive dysfunctions seen in many ill patients. Note that this paper is a preprint and corrections may be made in the future.
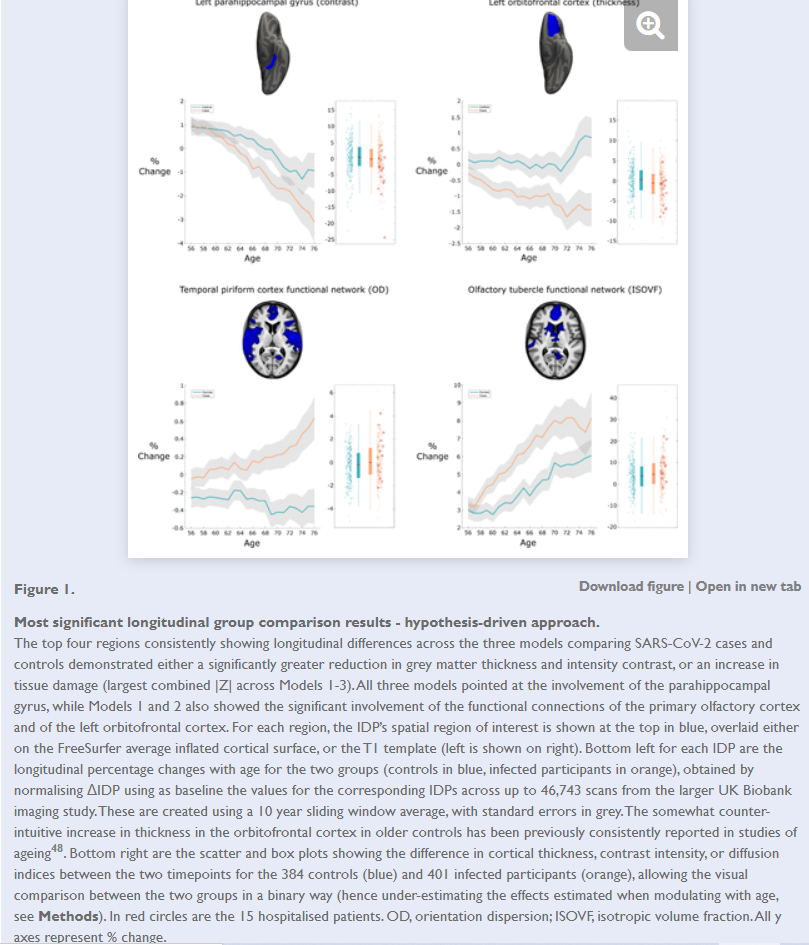
Adding to our previously established hypothesis, the authors noted a possible relationship between the loss of smell and the viral route through the olfactory bulb (emphasis mine):
Early neurological signs in COVID-19 include hyposmia and hypogeusia, which appear to precede the onset of respiratory symptoms in the majority of affected patients2, 18, 58. In addition, a heavily-debated hypothesis has been that an entry point of SARS-CoV-2 to the central nervous system is via the olfactory mucosa, or the olfactory bulb2, 11, 18. (The coronavirus itself would not necessarily need to enter the central nervous system; anterograde degeneration from olfactory neurons might suffice to generate the pattern of abnormalities revealed in our longitudinal analyses.) The predominance observed in other studies of hyposmic and anosmic symptoms — whether caused directly by loss of olfactory neurons or by perturbation of supporting cells of the olfactory epithelium15, 20 — could also, through repeated sensory deprivation, lead to loss of grey matter in these olfactory-related brain regions. Very focal reduction in grey matter in the orbitofrontal cortex and insula have been observed for instance in patients with severe olfactory dysfunction in a cross-sectional study of chronic rhinosinusitis26. A more extensive study of congenital and acquired (post-infectious, chronic inflammation due to rhinosinusitis, or idiopathic) olfactory loss also demonstrated an association between grey matter volume and olfactory function in the orbitofrontal cortex17. It also showed that duration of olfactory loss for those with acquired olfactory dysfunction, ranging from 0 to over 10 years, was related to more pronounced loss of grey matter in the gyrus rectus and orbitofrontal cortex17. On the other hand, it has been reported in a longitudinal study that patients with idiopathic olfactory loss had higher grey matter volume after undergoing olfactory training in various brain regions including the orbitofrontal cortex and gyrus rectus59. This raises the interesting possibility that the pattern of longitudinal abnormalities observed here in the limbic, olfactory brain regions of SARS-CoV-2 positive participants, if they are indeed related to olfactory dysfunction, might be attenuated over time if the infected participants go on to recover their sense of smell and taste.
So we have evidence of the neuroinvasive route SARS-COV2 can take through the olfactory nerves, and how that may contribute to the anosmia and the neurocognitive impairments such as brain fogs and delusions that may become present during an infection.
Why do Children Fare Better?
One of the strangest occurrences during the pandemic has been the very low rates of infection and death in children compared to adults. There are many social circumstances that may indicate why that may be, considering that many children stayed home for more than a year and had greatly reduced social interactions. However, we won’t concern ourselves with social considerations, and will focus on biological circumstances, particularly the nasal cavity.
One of the largest factors in lower infection rates among children may be due to nasal ACE2 expression.
In a study conducted by Bunyavanich et. al. 2020 researchers conducted a retroactive nasal epithelium examination where samples collected from patients ages 4-60 were sequenced for ACE2 gene expression. The results indicated an age-dependent expression of ACE2 in nasal epithelium.
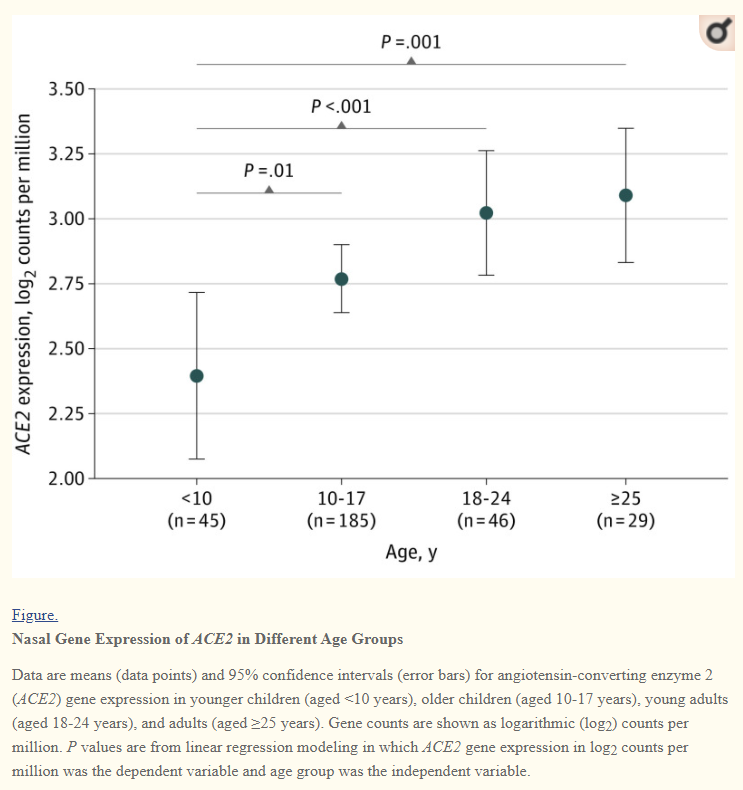
This study highlights a very important relationship. Higher nasal ACE2 expression could be indicative of greater viral entryway into host nasal epithelial cells and thus greater nasal viral load, all of which may lead to greater presentations of COVID symptoms.
As previously indicated, higher innate immunity may play an important factor in reducing viral infection, and there’s evidence of higher nasal innate immunity in children. In a study conducted by Pierce et al. 2021, innate immune gene expression was examined between children and adults, with the researchers finding greater innate immune response in children.
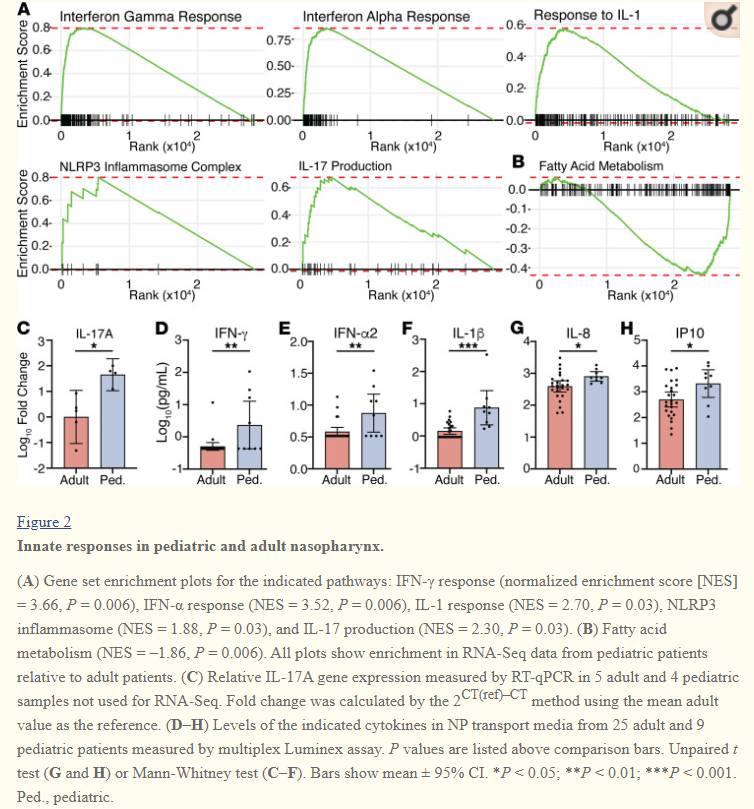
As the authors noted:
Our data suggesting increased expression of genes that are markers of immune cells including B and T cells and possibly antigen-presenting cells in NP swabs obtained from pediatric compared with adult patients is consistent with an enhanced immune response. The increase in CD20 expression as well as the inverse relationships between Ig gene expression and clinical outcomes suggest that a robust B cell response in the nasopharynx is protective.
Note that the researchers indicated higher ACE2 nasal expression in children within their cohort, which may be a concern due to the small sample size.
Nevertheless, it seems that there may be a direct relationship between nasal ACE2 expression and disease severity, and that an inverse relationship exists between nasal innate immunity and disease severity. More studies will help to further elucidate these relationships, but this helps to highlight the importance of the nasal environment as it is the first route of viral entry. In this case, the difference between gene and immune expression between adults and children may contribute to the differences in disease severity and death rates.
The Nose: Complexity in Simplicity
Our noses seem like one of the most simple structures, and yet its functions contribute greatly to our everyday lives. It provides us with the ability to take in external stimuli through smell and taste, and it provides one of the first barriers to infection. When we lose our ability to smell or begin to have a stuffy nose we should examine the causes, and what those causative agents can tell us about the relationship between our bodies and foreign pathogens.
The information we’ve outlined above illustrates a compelling scenario with respect to COVID infections. SARS-COV2 viral entry into the body predominately occurs through the nose. Once there, large amounts of viral replication begins to occur due to the large expression of ACE2 receptors.
This creates a serious predicament; nasal viral clearance may depend more on innate immunity rather than adaptive immunity, meaning that large levels of viral replication can occur and will not be seriously dealt with until the virus gains greater circulation within the body where the adaptive immune system may more effectively clear the infection. This would fit with a previous Substack post where I indicated that fully vaccinated individuals who had PCR tests done had extremely low Ct counts in the high single/low teens, suggesting that a trained adaptive immune system may not be able to deal with viral clearance within the nasal cavity. At the same time, the proximity of the olfactory bulb may mean that neuroinvasion may occur extremely early within the disease progression, which would align with the people who may experience mild illness but still experience anosmia, and we have also indicated changes in grey matter in the brain may contribute to neurocognitive changes such as brain fog and possible delusions.
The distinction between severity of disease between adults and children also highlights the importance in examining the environment where the initial viral infection occurs. Greater nasal innate immunity in children adds to the notion that innate immunity may be a more vital player in regards to initial infection. Also, the possibility of reduced ACE2 expression in children means that reduced viral entry may reduce the ability for the virus to replicate.
This piece outlines a critical role the nasal passage plays in viral progression, and this information may help elucidate how to treat SARS-COV2 infections. Treatments may need to be considered far earlier than typical presentation of symptoms such as dry coughs or fever, and prophylaxis that can be administered intranasally may prove to be far more effective than orally administered prophylaxis which may not be able to access the nasal cavity region of the body.
In my next Substack, which will be my first paid subscribers only piece, I will outline various intranasal therapeutic reigmens, including intranasal vaccine administration as well as intranasal prophylaxis.
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.
Edit: I sprinkled in a few more Subscribe buttons throughout the newsletter.



